 References in PDF file.
References in PDF file.
Master A, Wójcicka A, Giżewska K, Popławski P, Williams GR, Nauman A. A Novel Method for Gene-Specific Enhancement of Protein Translation by Targeting 5’UTRs of Selected Tumor Suppressors. PLoS ONE. 2016 May 12; 11(5): e0155359. Click the link.
Koronowicz AA, Kopeć A, Master A, Smoleń S, Piątkowska E, Bieżanowska-Kopeć R,Ledwożyw-Smoleń I, Skoczylas Ł, Rakoczy R, Leszczyńska T, Kapusta-Duch J, Pysz M.Transcriptome Profiling of Caco-2 Cancer Cell Line following Treatment with Extracts from Iodine-Biofortified Lettuce (Lactuca sativa L.). PLoS One. 2016 Jan 22;11(1):e0147336. Click the link.
Wojcicka A, Piekielko-Witkowska A, Kedzierska H, Rybicka B, Poplawski P, Boguslawska J, Master A, Nauman A. Epigenetic regulation of thyroid hormone receptor Betain renal cancer. PLoS One. 2014 May 21;9(5):e97624. Click the link.
Master A, Nauman A. THRB (Thyroid Hormone Receptor, Beta). Atlas Genet Cytogenet Oncol Haematol. 2014; 18(6). This article was published as online updated version in November 2013 and is available under the following links: XPS (recommended); Article–HTML; Article–PDF; AGCOH no. 06, 2014 (click with CTRL).
Master A, Nauman A. Molecular mechanisms of protein biosynthesis initiation - biochemical and biomedical implications of a new model of translation enhanced by the RNA hypoxia response element (rHRE) [Molekularne mechanizmy inicjacji biosyntezy białek - biochemiczne i biomedyczne implikacje nowego modelu translacji wzmacnianej przez element RNA odpowiedzi na hipoksję (rHRE)].
Postepy Biochem. 2014;60(1):39-54. Review. XPS (recommended), PDF or Journal-link.
Master A, Nauman A. Gene expression regulation by long naturally occurring antisense transcripts [Regulacja ekspresji genów przez długie naturalnie występujące antysensowne transkrypty]. Post. Biol. Kom. 2014;41(1):3-28. Review. XPS, PDF, Journal-link-1 or Journal-link-2.
Master A, Nauman A. Genomic context and expression regulation of nuclear thyroid hormone receptors by long naturally occurring antisense transcripts [Genomowy kontekst i regulacja ekspresji receptorów hormonu tarczycy przez długie naturalnie występujące antysensowne transkrypty]. Post. Biol. Kom. 2014; 41(1):29-58. Review. XPS, PDF, Journal-link-1 or Journal-link-2.
Boguslawska J, Wojcicka A, Piekielko-Witkowska A, Master A, Nauman A. MiR-224 targets the 3'UTR of type 1 5'-iodothyronine deiodinase possibly contributing to tissue hypothyroidism in renal cancer. PLoS One. 2011;6(9):e24541. Click the link.
Master A, Wójcicka A, Piekiełko-Witkowska A, Bogusławska J, Popławski P, Tański Z, Darras VM, Williams GR, Nauman A. Untranslated regions of Thyroid hormone receptor beta 1 mRNA are impaired in human clear cell renal cell carcinoma. Biochim Biophys Acta. 2010 Nov;1802(11):995-1005. Html-link; PDF-link.
Piekielko-Witkowska A, Master A, Wojcicka A, Boguslawska J, Brozda I, Tanski Z, Nauman A. Disturbed expression of type 1 iodothyronine deiodinase splice variants in human renal cancer. Thyroid. 2009 Oct;19(10):1105-13. Click link.
Nauman A, Piekiełko – Witkowska A, Turowska O, Popławski P, Master A, Tański Z, Lampkowska J, Wójcicka A, Brózda I, Puzianowska – Kuźnicka M. Disturbances of thyroid hormone signal pathway – in clear cell renal cell carcinoma [Zaburzenia szlaków sygnałowych hormonu tarczycy -trijodotyroniny - w raku nerki typu jasnokomórkowego]. Postepy nauk medycznych. 2008, 5: 268-278. Click the link.
Wisniewski P, Master A, Kaminska B. Cloning and purification of functionally active Fas ligand interfering protein (FIP) expressed in Escherichia coli. Acta Biochim Pol. 2008;55(1):51-6. Epub 2008 Jan 18. Click the link.
Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, Franciszkiewicz K, Chouaib S, Kaminska B. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion—an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008 Feb 7;27(7):918-30. Epub 2007 Aug 6. Click the link.
Nauman A, Turowska O, Poplawski P, Master A, Tanski Z, Puzianowska-Kuznicka M. Elevated cyclin E level in human clear cell renal cell carcinoma: possible causes and consequences. Acta Biochim Pol. 2007;54(3):595-602. Epub 2007 Aug 28. Click the link.
Turowska O, Nauman A, Pietrzak M, Popławski P, Master A, Nygard M, Bondesson M, Tanski Z, Puzianowska-Kuznicka M. Overexpression of E2F1 in clear cell renal cell carcinoma: a potential impact of erroneous regulation by thyroid hormone nuclear receptors. Thyroid. 2007 Nov;17(11):1039-48. Click the link.
Switaj K, Master A, Borkowski PK, Skrzypczak M, Wojciechowicz J, Zaborowski P. Association of ocular toxoplasmosis with type I Toxoplasma gondii strains: direct genotyping from peripheral blood samples. J Clin Microbiol. 2006 Nov;44(11):4262-4. Click the link.
Switaj K, Master A, Skrzypczak M, Zaborowski P. Recent trends in molecular diagnostics for Toxoplasma gondii infections. Clin Microbiol Infect. 2005 Mar;11(3):170-6. Review. link.
Madeja Z, Master A, Michalik M, Sroka J. Contact-mediated acceleration of migration of melanoma B16 cells depends on extracellular calcium ions. Folia Biol. 2001;49(3-4):113-24. Click the link.
SELECTED ABSTRACTS:
Master A1, Wojcicka A2, Nauman A1. The 5’UTR-dependent enhancement of protein translation efficiency triggered by self-transfecting 3’-aminoallyl-containing oligonucleotides (aa-dGoligos) targeting a pool of strongly folded transcript variants of the THRB suppressor gene. Molecular Therapy Volume 22, Supplement 1, May 2014 (Nature Publishing Group). The 17th Annual Meeting of the American Society of Gene & Cell Therapy, May 21-24, 2014 Washington, DC, USA. Session: Gene Targeting and Gene Correction I. 1The Centre of Postgraduate Medical Education, Department of Biochemistry and Molecular Biology, ul. Marymoncka 99/103, 01-813 Warsaw, Poland. 2Genomic Medicine, Depart. of General, Transplant and Liver Surgery, Medical University of Warsaw, ul. Żwirki i Wigury 61, 02-091 Warsaw, Poland. To see click the poster-link or abstract-link.
Koronowicz A1, Master A2, Sikora E1, Leszczynska T1, Dulinska-Litewka J3. The influence of fatty acids of egg yolks enriched with CLA on mRNA expression of TP53, CASP3 i MYC genes. [Wpływ mieszaniny kwasów tłuszczowych z żółtka wzbogaconego w CLA na ekspresję mRNA genów: TP53, CASP3 i MYC]. 1) University of Agriculture in Cracow, Faculty of Food Technology, Poland, 2) Centre of Postgraduate Medical Education, Department of Biochemistry and Molecular Biology, Warsaw, Poland, 3) Jagiellonian University, Collegium Medicum, Department of Medical Biochemistry. XXIII Polish Bromatological Symposium „Bromatology for XXI Century Society”. Cracow 10-12th of September 2014r.
Domagala D1, Koronowicz A1, Master A2, Dulinska T4, Leszczynska T1. Antagonistic effect of CLA isomers on expression of selected cell cycle genes in breast cancer cell line T47D. 1) Faculty of Food Technology, University of Agriculture in Cracow, Poland; 2) The Centre of Postgraduate Medical Education, Department of Biochemistry and Molecular Biology, Warsaw, Poland; 3) Department of Medical Biochemistry, Jagiellonian University, Collegium Medicum, Krakow, Poland. Abstract (1835) accepted for presentation at FEBS-EMBO congress, Paris, France from 30 August - 4 September 2014.
Koronowicz Aa, Master Ab, Chwastek Ja, Szymczyk Bc Leszczynska Ta. The influence of fatty acids of egg yolks enriched with CLA on activation of PPAR receptors in prostate cancer cells [Wpływ kwasów tłuszczowych z żółtek jaj wzbogaconych w CLA na aktywację receptorów PPAR w komórkach rakowych gruczołu krokowego]. XLI session of Food Science Committee of Polish Academy of Science, Cracow, 2-3th of July 2013. Session: Modern techniques and technologies in food processing and food conservation. a) University of Agriculture in Cracow, Faculty of Food Technology, Poland, b) Centre of Postgraduate Medical Education, Department of Biochemistry and Molecular Biology, Warsaw, Poland, c) Faculty of Animal Feeding and Technology, University of Agriculture in Cracow, Poland, and National Research Institute of Animal Production, Department of Animal Nutrition and Feed Science, Poland.
Boguslawska J, Piekielko-Witkowska A, Wojcicka A, Master A, Nauman A, Mir-224 contributes to tissue hypothyroidism in renal cancer, targeting the 3'UTR of type 1 5´-iodothyronine deiodinase, 35th Meeting of the European Thyroid Association, 10-14.09.2011 Krakow, European Thyroid Journal, Launching Issue, p. 78.
Piekiełko-Witkowska A, Master A, Wojcicka A, Bogusławska J, Wiszomirska H, Tanski Z, Darras VM, Williams GR, Nauman A. Zaburzenia ekspresji receptora hormonu tarczycy TRβ1 w raku nerki człowieka”. 2 Zjazd Polskiego Towarzystwa Tyreologicznego (2d Meeting of Polish Thyroid Association), 20-22.05.2010 Zakopane, Endokrynologia Polska tom 61 nr 5, 2010, str 578.
Bogusławska J, Master A, Piekiełko-Witkowska A, Wójcicka A, Popławski P, Tański Z, Nauman A. Rola mikroRNA w zaburzeniach ekspresji jodotyroninowej dejodynazy typu I w raku nerki typu jasnokomórkowego (ccRCC). 2 Zjazd Polskiego Towarzystwa Tyreologicznego (2d Meeting of Polish Thyroid Association), 20-22.05.2010 Zakopane, Endokrynologia Polska tom 61 nr 5, 2010, str 578.
Boguslawska J, Master A, Wojcicka A, Popławski P, Piekielko-Witkowska A, Nauman A. The Role of miRNA in reduction of type I 5’-iodothyronine deiodinase expression (D1) in renal clear cell carcinoma (ccRCC). 12-th European Congress of Encocrinology 24-29.04.2010 Prague, Czech, Endocrine Abstracts 2010, 22.
Wójcicka A, Piekiełko-Witkowska A, Popławski P, Master A, Lampkowska J, Brózda J, Tański Z, Nauman A. Potencjalna rola metylacji w wyciszaniu ekspresji genu receptora jądrowego hormonu tarczycy TRbeta w raku nerki typu jasnokomórkowego. 1 Zjazd Polskiego Towarzystwa Tyreologicznego, 26-28.03.2009 Szczecin, Endokrynologia Polska, 2009; 60; supl.A; 3-4.
Bogusławska J, Master A, Piekiełko-Witkowska A, Wójcicka A, Popławski A, Brózda I, Tański Z, Nauman A. Rola 3’UTR I mikroRNA w zaburzeniach ekspresji jodotyroninowej dejodynazy typu I w raku nerki typu jasnokomórkowego (ccRCC). 1 Zjazd Polskiego Towarzystwa Tyreologicznego, 26-28.03.2009 Szczecin, Endokrynologia Polska, 2009; 60; supl.A; 3-4.
Boguslawska J, Master A, Piekielko-Witkowska A, Wojcicka A, Brozda I, Poplawski P. Macke-Nauman A. Reduction of type I 5'-iodothyronine deiodinase activity and mRNA level in renal clear cell carcinoma (ccRCC): potential role of 3' UTR and miRNA. BES 15-19.03.2009, Harrogate, Great Britain.
Boguslawska J, Master A, Piekielko-Witkowska A, Wojcicka A, Poplawski P, Nauman A. The micro RNA targets the 3’UTR of type I 5’-iodothyronine deiodinase resulting in the reduction of D1 mRNA level in renal clear cell carcinoma (ccRCC). EMBO workshop Messenger RNA 3’ ends & gene expression, 16-20.09.2009 Oxford, Great Britain.
Master A1, Wojcicka A1, Poplawski P1, Piekielko-Witkowska A1, Boguslawska J1, Brozda I1, Wiliams GR2 and Nauman A1. Short oligodeoxynucleotides (dgoligos) targeting of the THRB gene 5'-UTR markedly increase the efficiency of TRβ protein translation - a therapeutic potential of sense/antisense based dGoligos as Gibb's free energy modulators of 5'-utr stem-loop structure. 05-09.09.2009. Lisbon, European Thyroid Association. Acta Medica Portugesa, 2009; 22; 4(11); s.13. The Young Investigators’ Prize. 1The Centre of Postgraduate Medical Education, Department of Biochemistry and Molecular Biology, ul. Marymoncka 99/103, 01-813 Warsaw, Poland. 2Molecular Endocrinology Group, Imperial College London, MRC Clinical Sciences Centre, Hammersmith Hospital, Du Cane Road, London, W12 0NN. Click the link.
Master A1, Piekielko-Witkowska A1, Poplawski P1, Lampkowska J1,Wojcicka A1, Brozda I1, Tanski Z2 Williams GR3,Alicja Nauman1. Aberrant expression of human thyroid hormone receptor b1 protein may be regulated by alternative untranslated regions of THRB mRNA in clear cell renal cell carcinoma (ccRCC). 1Department of Biochemistry and Molecular Biology, Medical Center of Postgraduate Education, Warsaw, Poland, 2Specialistic Hospital, Ostroleka, Poland, 3Molecular Endocrinology Group, Imperial College London, MRC Clinical Sciences Centre, Hammersmith Hospital, Du Cane Road, London, W12 0NN. The European Thyroid Association (ETA), Thessaloniki 2008. Click the link.
Piekiełko-Witkowska A, Master A, Wojcicka A, Lampkowska J, Poplawski P, Brozda I, Tanski Z, Nauman A. Disturbed alternative splicing of type 1 deiodinase in renal cancer: potential role of splicing factors SF2/ASF and hnRNPA1” 33d Meeting of European Thyroid Association, 20-24.09.2008 Chalkidiki-Thesaloniki, Greece, Hormones/IJEM International Journal of Endocrinology and Metabolism, Vol 7, Supl 1, 2008.
Piekiełko-Witkowska A, Popławski P, Master A, Lampkowska J, Wojcicka A, Brozda I, Nauman A. The expression and triiodothyronine dependent regulation of two splicing factors: SF2/ASF and hnRNPA1 is disturbed in clear cell renal cell carcinoma, EMBO Conference Series, RNA and Disease, RNA Metabolism and Associated Pathologies, 31.05-06.06.2008 Rome, Italy.
Piekiełko-Witkowska A, Master A, Wojcicka A, Lampkowska J, Poplawski P, Brozda I, Tanski Z, Nauman A. Regulacja jodotyroninowej dejodynazy typu 1 na poziomie posttranskrypcyjnym w raku nerki typu jasnokomórkowego. Potencjalna zwrotna regulacja alternatywnego splicingu przez trijodotyroninę. 19 kongres Polskiego Towarzystwa Endokrynologicznego, 25-28.09.2008 Wrocław, Nagroda Polskiego
Towarzystwa Endokrynologicznego, Endokrynologia Polska, tom 59, supl. A, 2008.
Lampkowska J, Piekiełko-Witkowska A, Master A, Wójcicka A, Popławski P, Brozda I, Nauman A. “Reduction of type I 5'-iodothyronine deiodinase activity and mRNA level in renal clear cell carcinoma. Alterations in 3’ UTR and miRNA expression”. EMBO „High-Throughput microRNA Profiling”, 7-12.04.2008 Heidelberg, Germany.
Master A1, Piekielko-Witkowska A1, Poplawski P1, Tanski Z2, Nauman A1. The expression of alternatively spliced 5’-UTR mRNA variants of Human Thyroid Hormone Receptor Beta 1 (THRB) is dependent on the tissue type and aberrant in clear cell renal cell carcinoma (ccRCC). Horm Res 2007;68 (Suppl. 3):48-49. 1The Centre of Postgraduate Medical Education, Department of Biochemistry and Molecular Biology, ul. Marymoncka 99/103, 01-813 Warsaw, Poland. 2Specialistic Hospital, Ostroleka, Poland.
Master A1, Piekielko-Witkowska A1, Poplawski P1, Tanski2, Alicja Nauman1. The expression of alternatively spliced 5’-UTR mRNA variants of Human Thyroid Hormone Receptor Beta 1 (THRB) is dependent on the tissue type and aberrant in clear cell renal cell carcinoma (ccRCC). 1Department of Biochemistry and Molecular Biology, Medical Center of Postgraduate Education, Warsaw, Poland, 2Specialistic Hospital, Ostroleka, Poland. The European Thyroid Association (ETA), Leipzig 2007. To see click the link.
Wesołowska A, Master A, Śliwa M, Kamińska-Kaczmarek B. Development of siRNA against TbRII blocking efficiently TGFb1 signalling pathways in glioma cells, The FEBS Journal, 2005, vol. 272, p48, Suppl. 1. Laboratory of Molecular Neurobiology, Nencki Institute of Experimental Biology, Polish Academy of Sciences, 3 Pasteur Street 02-093 Warszawa, Poland.
Master A1, Świtaj K2, Skrzypczak M1, Zaborowski P2, Płucienniczak A1. Genetic diagnostics of Toxoplasma gonidii [Diagnostyka genetyczna Toxoplasma gonidii]. 1The Institute for DNA Research, Warsaw, ul. Rakowiecka 36, 2Department of Zoonotic and Tropical Diseases, Institute of Infectious and Parasitic Diseases, Medical University of Warsaw, ul. Wolska 37, Warsaw, Poland. XV annual meeting of Polish Society of Genetics and IV meeting of Polis Society of Human Genetics, 2004. Click the link.
SELECTED PATENTS:
Master Adam, Skrzypczak Magdalena, Master Aneta, Nowacka Joanna, Plucienniczak Andrzej, Wroblewska Sylwia (authors). Oligonucleotide and polynucleotide for the detecting and determining mutation, particularly hereditary mutation in the p53 human gene, method for the manufacture of this polynuclotide and method and the apparatus for the detecting and determining mutation. Patent number: PL373443 (A1). International classification: C12N15/10; C12P19/30; C12Q1/68; C12P35/00. Application number: PL20050373443 20050307. Publication date: 2006-09-18.
Master Adam, Skrzypczak Magdalena, Master Aneta, Nowacka Joanna, Plucienniczak Andrzej, Wroblewska Sylwia (authors). Oligonucleotide and polynucleotide for the detecting and determining mutation, particularly hereditary mutation in the human genes BRCA1 and BRCA2, method for the manufacture of this polynuclotide and method and the apparatus for the detecting and determining mutation in the human genes BRCA1 and BRCA2. Patent number: PL373442 (A1). International classification: C12N15/10; C12P19/30; C12N15/07; C12N15/11; A61P35/00. Application number: PL20050373442 20050307. Publication date: 2006-09-18.
Master Adam, Skrzypczak Magdalena (authors). Oligonucleotide, its application and method and apparatus designed for detection of gondiae DNA Toxoplasma gonidii. Patent number: PL358894 (A1). International classification: C07K14/45; C12Q1/68; C12P19/34; A61P33/02; C12N15/30. Application number: PL20030358894 20030226 . Publication date: 2004-09-06.
Participation in research GRANTS:
- 2012-2016; Research project: Conjugated linoleic acid (CLA)-induced transcriptional activation of PPAR-an investigation of molecular mechanisms of putative anticancer action of fatty acids of CLA-enriched egg yolks.This work is supported by the National Science Centre for Scientific Research Grants UMO-2011/03/B/NZ9/01423.
- 2009-2015; Research project: Structural and functional analysis of splice variants of TRβ1 in Human Renal Cancer. This work was supported by the Polish State Committee for Scientific Research Grants N N401 073636, N401 017 32/0286 (to A.N), and the Medical Centre of Postgraduate Education Grant 501-2-1-22-15/06. Realized in Medical Centre for Postgraduate Education in Warsaw, Poland.
- 2009-2011, Large project: Expression of genes involved in human epilepsy (research using microarray technology), coordinated by Adam Master at Laboratory of Molecular Medical Biology of BioTe21 in cooperation with Medical University in Lublin, Poland and Ocean Ridge Biosciences, Palm Beach Gardens, FL USA.
- 2006-2011, Project: Gene synthesis using the method of oligonucleotide assembly in PCR reaction, co-financed by the European Union from the European Regional Development Fund (ERDF) as part of the Integrated Regional Operational Programme (IROP). Adam Master’s project realized in Laboratory of Nucleic Acid Synthesis and Sequencing of BioTe21 in Cracow, Poland. The structural fund of EU allowed to provide the best quality, the most advanced research tools and high-resolution apparatus enable of performing the synthesis and analysis at genome and transcriptome level (ABI 3900 High-Throughput DNA Synthesizer, ABI 3100 Automated Capillary DNA Sequencer, HPLC, lyophilizer, thermocyclers and other research tools. Project partner: AME Bioscience Ltd, UK.
- 2008-2010, Project: Genetic diagnostics of inherited mutations of genes involved in melanoma malignum, realized in Laboratory of Molecular Medical Biology of Biote21, supported by the PKO BP.
- Project: N N401 3547 33, realized in Medical Centre for Postgraduate Education in Warsaw, Poland.
- Project N401 017 32/02863, realized in Medical Centre for Postgraduate Education in Warsaw, Poland.
- Project 501-2-1-24-06/08, realized in Medical Centre for Postgraduate Education in Warsaw, Poland.
- Project 501-2-1-24-07/08, realized in Medical Centre for Postgraduate Education in Warsaw, Poland.
- Project 501-2-1-22-15/06: realized in Medical Centre for Postgraduate Education in Warsaw, Poland.
- Participation in projects coordinated by AKCENT MALOPOLSKA, a consortium of Jagiellonian University, Cracow University of Technology, University of Agriculture, AGH University of Science and Technology, focused on Research programme, that covers the following priority areas: Biotechnology, Nanotechnology and Modern technologies in medicine. The consortium aims at realization of many-year interdisciplinary research and training programmne as well as efficient implementation and commercialization of modern technologies by the Universities in Cracow.
- Participation in an international proposal: Betacarotene metabolism as the risk of liver fat accumulation. Search for the new NAFLD risk markers, created for 7th EU Frame Programme in Health priority, coordinated by Collegium Medicum at Jagiellonian University.

Last figures from oryginal papers (drawn by Adam Master):
Master A, Nauman A.
Molecular mechanisms of protein biosynthesis initiation - biochemical and biomedical implications of a new model of translation enhanced by the RNA hypoxia response element (rHRE)
Postepy Biochem. 2014;60(1):39-54. Review. Click a link: XPS (recommended), PDF or Journal-link.
Abstract: Translation initiation is a key rate-limiting step in cellular protein synthesis. A cap-dependent initiation is the most effective mechanism of the translation. However, some physiological (mitosis) and pathological (oxidative stress) processes may switch the classic mecha-nism to an alternative one that is regulated by an mRNA element such as IRES, uORF, IRE, CPE, DICE, AURE or CITE. A recently discovered mechanism of RNA hypoxia response element (rHRE)-dependent translation initiation, may change the view of oxygen-regulated translation and give a new insight into unexplained biochemical processes. Hypoxia is one of the better-known factors that may trigger an alternative mechanism of the translation initiation. Temporal events of oxygen deficiency within tissues and organs may activate processes such as angiogenesis, myogenesis, regeneration, wound healing, and may promote an adaptive response in cardiovascular and neurodegenerative diseases. On the other hand, growth of solid tumors may be accompanied by cyclic hypoxia, allowing for synthesis of proteins required for further progression of cancer cells. This paper provides a review of current knowledge on translational control in the context of alternative models of translation initiation. Keywords: 5’ cap (m7G), cancer, IRES, HIF-2α, hypoxia, oxidative stress, rHRE, transla-tional control, UTR. Abbreviations: eIF, eukaryotic translation Initiation Factor; eIF4E2, eukaryotic transla-tion Initiation Factor 4E type 2 encoded by the EIF4E2 gene (NCBI Gene ID: 9470); HIF-2α, Hypoxia-Inducible Factor 2-alpha en-coded by the EPAS1 gene (Gene ID: 2034) that functions as a transcription factor and translation initiation factor activated under hypoxia conditions; IRES, Internal Ribosome Entry Site, an RNA element; ITAF, IRES Trans-Acting Factor, a ribonucleoprotein that can recognize the IRES cis-element in RNAs; 5’-cap, 7-methyloguanosine (m7G), that is bound to 5’-end of an mRNA during transcription; RBM4, RNA-binding protein 4, encoded by the RBM4 (Gene ID: 5936), the protein can bind to rHRE element; rHRE, RNA hypoxia response element, which can be found in multiple mRNAs; uORF, alterna-tive upstream Open Reading Frame, located upstream of the main ORF translation start site, between start and stop codons; UTR, UnTranslated Region, an RNA fragment that is found on the 5’ (5’UTR) or 3’-side (3’UTR) of mRNAs. Acknowledgments: This work was supported by the National Science Centre (NCN) research grants no.: N N411073636 and NN 401611940. The article is a part of Adam Master’s PhD dissertation entitled: “The role of untranslated regions (UTRs) in expression of Thyroid Hormone Receptor Beta 1 isoform (TRβ1) in human clear cell Renal Cell Carcinoma (ccRCC).”  Fig.1A. Model of 5’ cap (m7G)-dependent initiation of translation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].
Fig.1A. Model of 5’ cap (m7G)-dependent initiation of translation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].
 Fig.1B.General model of IRES-dependent (5’ cap- independent) initiation of translation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].
Fig.1B.General model of IRES-dependent (5’ cap- independent) initiation of translation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].
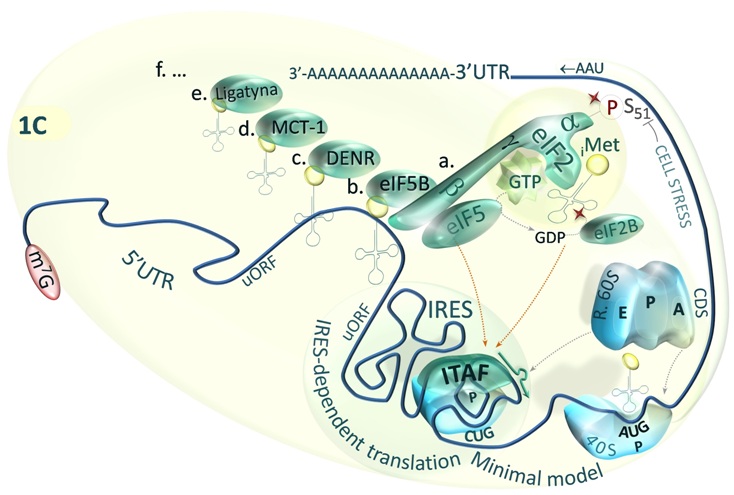
Fig.1C. Minimal model of IRES-dependent initiation of translation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].

Fig.1D. Model of rHRE-dependent initiation of translation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].
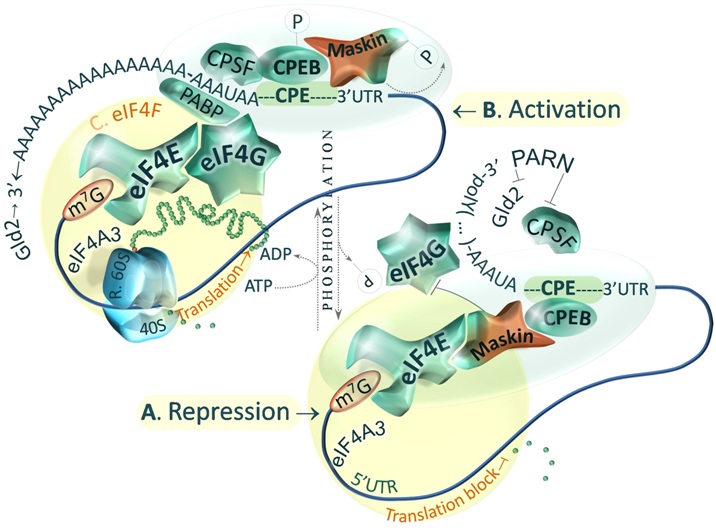 Fig.2. Model of Maskin-mediated control of translation activation and initiation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].
Fig.2. Model of Maskin-mediated control of translation activation and initiation [Master & Nauman, Postepy Biochem. 2014;60(1):39-54].
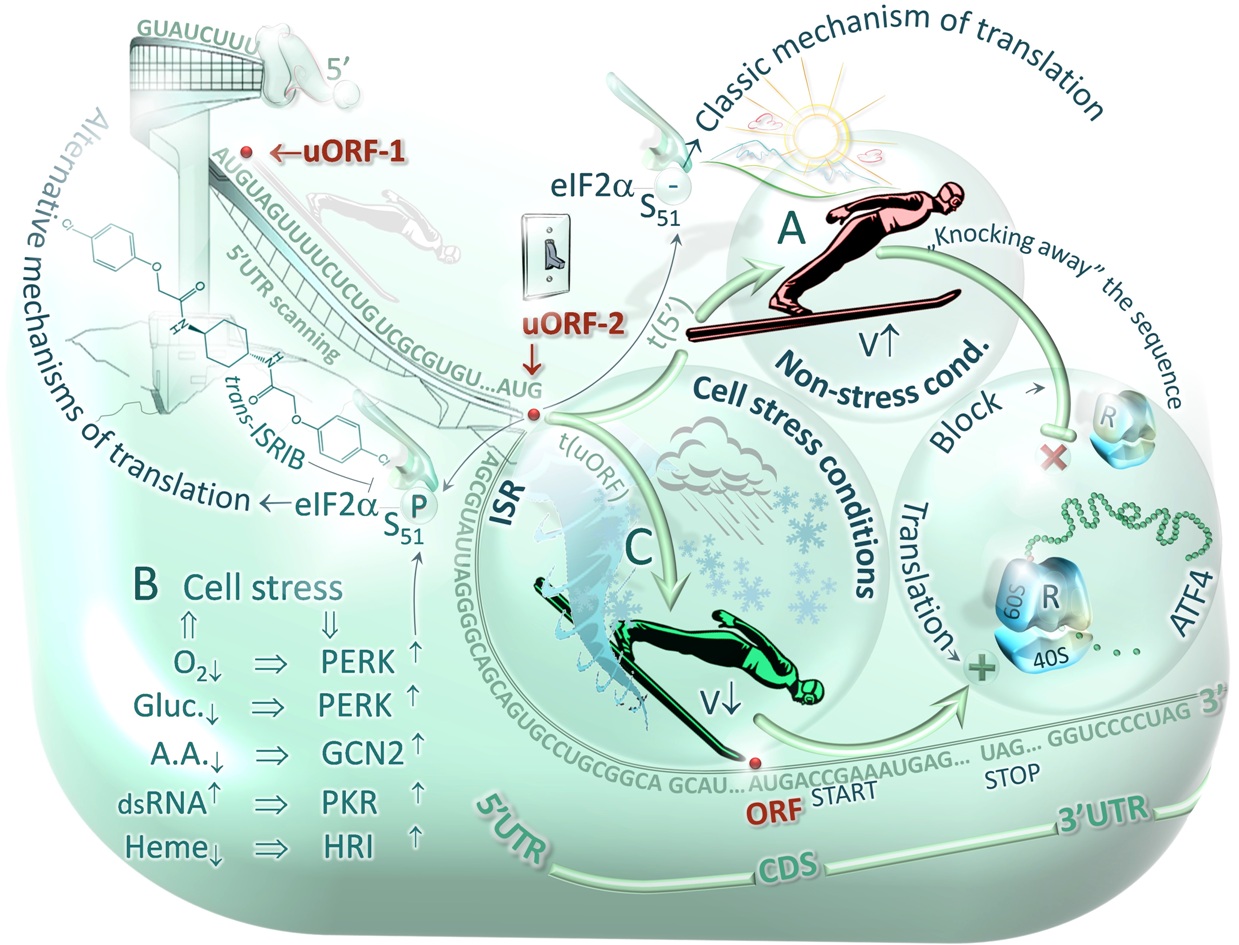
Fig.3. Scheme of stress-activated (B) protein kinases -mediated switching of alternative mechanisms of: cap-dependent (A) and uORF-dependent translation (C) [Master & Nauman, Postepy Biochem. 2014;60(1):39-54]
Master A, Nauman A.
Gene expression regulation by long naturally occurring antisense transcripts.
Post. Biol. Kom. 2014;41(1):3-28. Review. Click a link: XPS (recommended), PDF, Journal-link-1 or Journal-lin
Summary: Human genome sequence contains information about structure and function of the whole or-ganism. However, specific features of individual cells and tissues are determined by gene expression regulation mechanisms, in which long naturally occurring antisense transcripts (NATs) may play a sub-stantial role, largely underestimated so far. These molecules show full or partial complementarity to func-tional genes and may control their expression on the level of RNA-directed DNA methylation, pre-mRNA transcription, alternative splicing as well as RNA editing, transport and localized protein translation. In contrast to a group of short non-coding RNAs (sncRNAs) such as microRNA, the NATs including long non-coding RNAs (lncRNAs) usually exhibit the higher complementarity, and hence the higher specificity towards target genes. Binding of these molecules to DNA or RNA may obstruct regulatory elements or influence the stability of some functional structures such as G-quadruplexes and internal ribosome entry sites (IRES elements). The new technologies of whole genome sequencing and screening of cDNA li-braries revealed that about 20% of human and mouse genes overlap resulting in formation of potential sense-antisense pairs. Though, the physiological relevance of most of the pairs remains obscure, there are a number of identified and annotated antisense transcripts that are known to regulate expression of genes involved in cell growth, proliferation, differentiation, apoptosis, metabolism and circadian cycle. NATs expression have been found to be aberrant in the course of various diseases including cancers that indicates the need for understanding the roles of these transcripts at each step in the flow of genetic in-formation. This paper provides a review of current knowledge on gene expression regulation by long RNAs showing NATs activity. Moreover, a definition as well as structural and functional characteristics of the heterogeneous group of transcripts has been proposed on the basis of the NATs’ ability to form sense-antisense pairs and the subsequent potential to activate mechanisms controlling target gene ex-pression. Keywords: cancer cells, G-quadruplex, long naturally occurring antisense transcripts - NATs, lncRNA, pseudogenes.
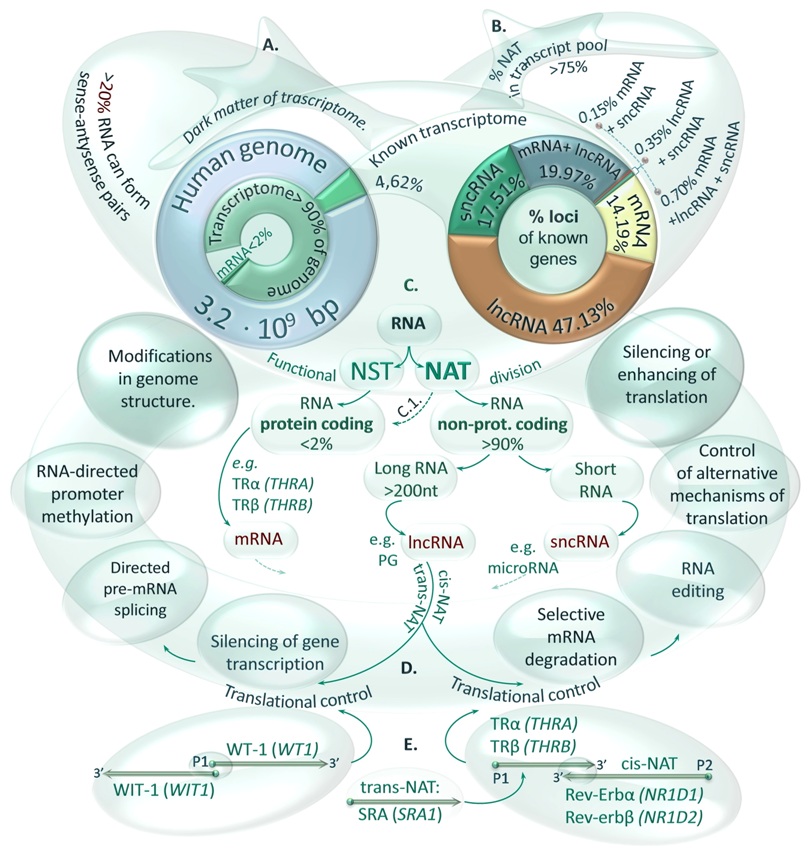
FIGURE 1. Functional and structural classification of transcripts (RNAs). A. Transcriptome size in the context of the human genome sequence. Although it is estimated that over 90% of the genome sequence can be transcribed, the cellular proteome is encoded by less than 2% of the genome. The biological functions of the most transcripts are unknown (dark matter of the transcriptome), however, it was calculated that at least 20% of cellular RNAs have the potential to form sense-antisense pairs. B. Participation of various RNA classes in an annotated part (4.62 %) of the human transcriptome. Percentage values of gene loci number occupied by 4 categories of transcripts are shown in right circle graph. C. Functional classification of RNA molecules. Transcripts were divided onto two groups of RNAs: naturally occurring sense (NST) and antisense (NAT) transcripts including lncRNAs and pseudogenes (PGs) – a class of non-protein coding or non-functional protein coding RNAs. Nevertheless, the function of NATs may be also fulfilled by protein coding mRNAs transcribed from overlapping genes such as THRA and NR1D1 encoding TRα2 and Rev-Erbα proteins. D. The flow of genetic information controlled by transcripts showing NAT’s activity. Complementarity of transcripts may be observed when their genes share the common sequence in the same locus (cis-NATs) or distant loci (trans-NATs) that may result from gene duplication during genome evolution. Furthermore, the long double-stranded RNAs (dsRNAs) can trigger specific responses at the level of transcription or translation. E. NATs’ binding to mRNAs. Cis- and trans-NATs can interact with various mRNA regions forming long RNA pairs such as WT-1 / WIT-1 transcribed from the same bidirectional promoter, TRα / Rev-Erbα with overlapping 3’UTR regions or SRA affecting the expression and function of the thyroid hormone receptors (TRs).
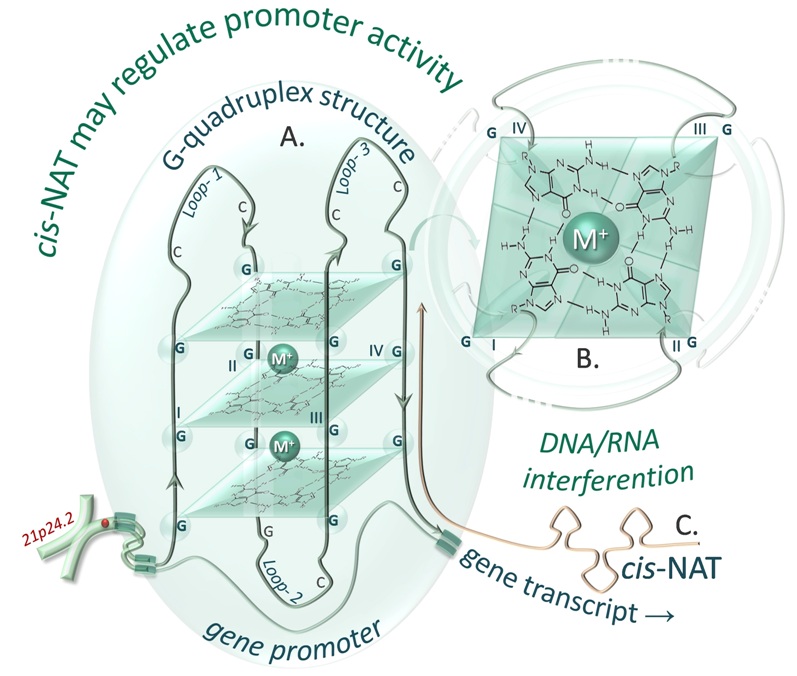
FIGURE 2. Scheme of interaction between NATs and G-quadruplex structures. A. Intermolecular G-quadruplex structure in an antiparallel conformation. The model was designed on the basis of a G-quadruplex consensus sequence found in silico in THRB gene promoter (GGGCCGGGGCGGGCCGGG). B. Magnification of a single G-quadruplex plain, cooperatively stabilized by a metal ion (M+) and hydrogen bonds of guanine (G). The G-quadruplex structure could be disturbed by a cis-NAT (e.g. LOC644990) that is complementary to this promoter region.
Master A, Nauman A.
Genomic context and expression regulation of nuclear thyroid hormone receptors by long naturally occurring antisense transcripts.
Post. Biol. Kom. 2014; 41(1):29-58. Review. Click a link: XPS (recommended), PDF, Journal-link-1 or Journal-link-2.
Summary: The sequencing of whole genomes revealed that most of the human DNA is transcribed, and less than 2% of the human genome codes for proteins, which means that the majority of the transcripts are non coding RNAs (ncRNAs), and their function remains to be established. The long (>200 bp) naturally occurring antisense transcripts (long NATs) constitute a heterogeneous class of cellular, usually non-coding RNAs, which share the functional ability to specifically bind complementary target strands of DNA or RNA molecules. These sense-antisense pairs may activate numerous mechanisms leading to altered expression of target genes, located at the same locus (cis-NAT) or at distant loci (trans-NAT). The activity of the long NATs may be shown by a long non-protein coding RNA (lncRNA), a pseudogene transcript, but also by a protein-coding RNA molecule (pcRNA) that can bind another RNA molecule. Recently recognized mechanisms of the NATs-controlled gene regulation may throw a new light on expression of THRA and THRB genes encoding nuclear receptors of thyroid hormone - triiodotyronine (T3). Transcription of the genes may result in numerous mRNA variants translated into several protein isoforms: TRα1, TRα2, TRα3, TRβ1, TRβ2 showing activity of ligand (T3)-dependent transcription factors and regulating expression of various genes engaged in cell cycle progression, proliferation, differentiation and metabolism. The THRB gene is also thought to be a suppressor, which expression is deregulated in many types of tumors. This paper provides a review of current knowledge on NATs-mediated regulation of gene expression, with a special consideration of the THRA and THRB genome context and sequence that was analyzed using bioinformatical tools. It presents also first results confirming in clear cell Renal Cell Carcinoma (ccRCC) the presence of newly identified NR1D2 transcript variants showing in silico antisense complementarity to THRB gene transcripts. Furthermore, Real-Time PCR measurements of selected pairs of complementary sense-antisense transcripts revealed in ccRCC decreased levels of thyroid hormone receptor mRNA - TRα2 isoform (THRA), accompanied by significantly elevated expression of antisense rev-Erbα (NR1D1). Similar negative rela-tion was noted between TRβ1 variant F1 (THRB) and rev-Erbβ variant G (NR1D2) - identified here for the first time in ccRCC. Keywords: long naturally occurring antisense transcripts - NATs, lncRNA, NR1D1, NR1D2, THRB, THRA, clear cell Renal Cell Carcinoma - ccRCC.
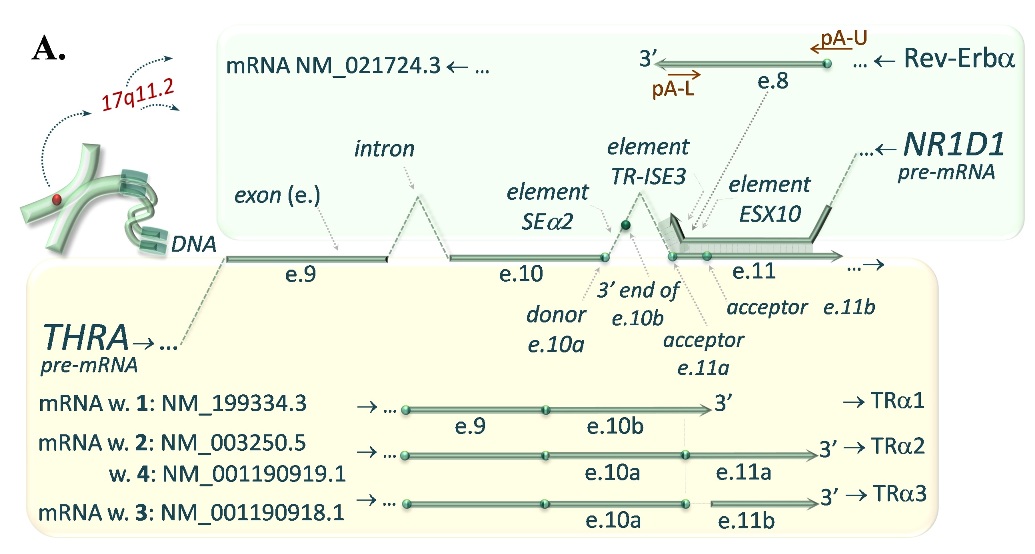
FIGURE 1A. Complementary interaction of transcripts encoded by the THRA and NR1D1 (A.) as well as THRB and NR1D2 (B.) genes. A. NATs-mediated regulation of gene expression in an instance of regulation of thyroid hormone receptor alpha (TRα) isoforms (encoded by THRA gene transcripts), which expression is controled by Rev-Erba (NR1D1 gene transcript). The complementarity between exon 8 (e.8) of the Rev-Erba (turquoise upper pannel) and exon 11a and 11b (e.11a, e.11b) of the TRα2 and TRα3 (yellow lower panel) may result in reduction of these mRNA variants in favour of the TRα1 isoform.
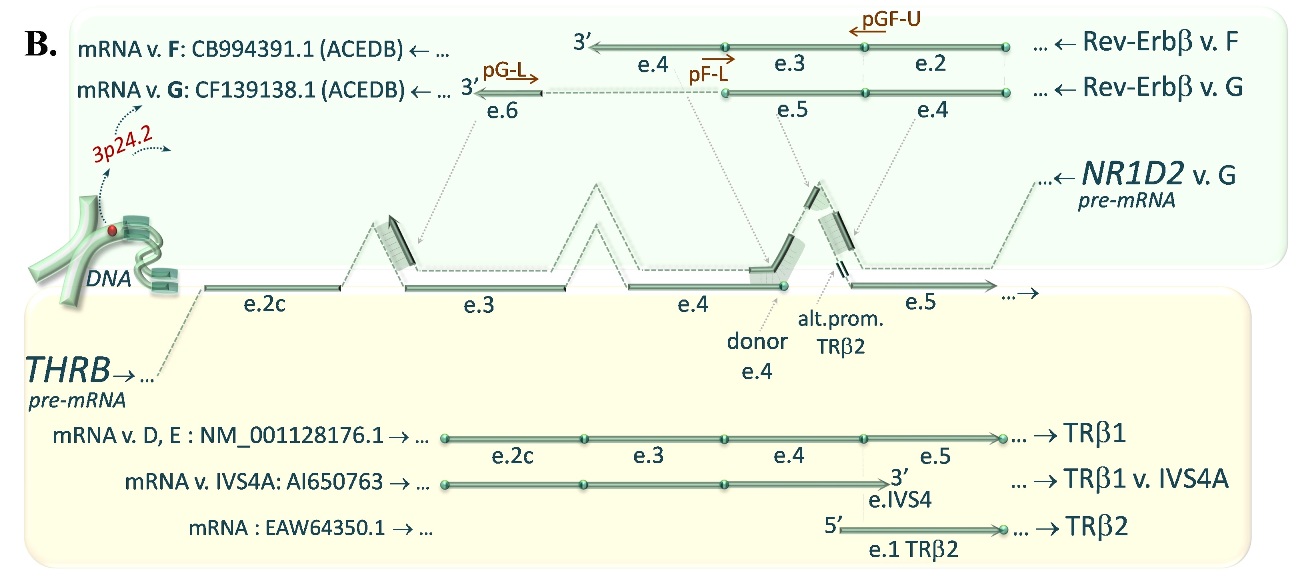
FIGURE 1B. The similar complementarity between 3’-ends of Rev-Erbβ variants F or G encoded by NR1D2 gene (turquoise upper pannel) and pre-mRNA of TRβ1, exon 4/5 junction site of the TRβ1 as well as promoter sequence of TRβ2 encoded by THRB gene (yellow lower panel) may affect the expression of the TRβ isoforms.
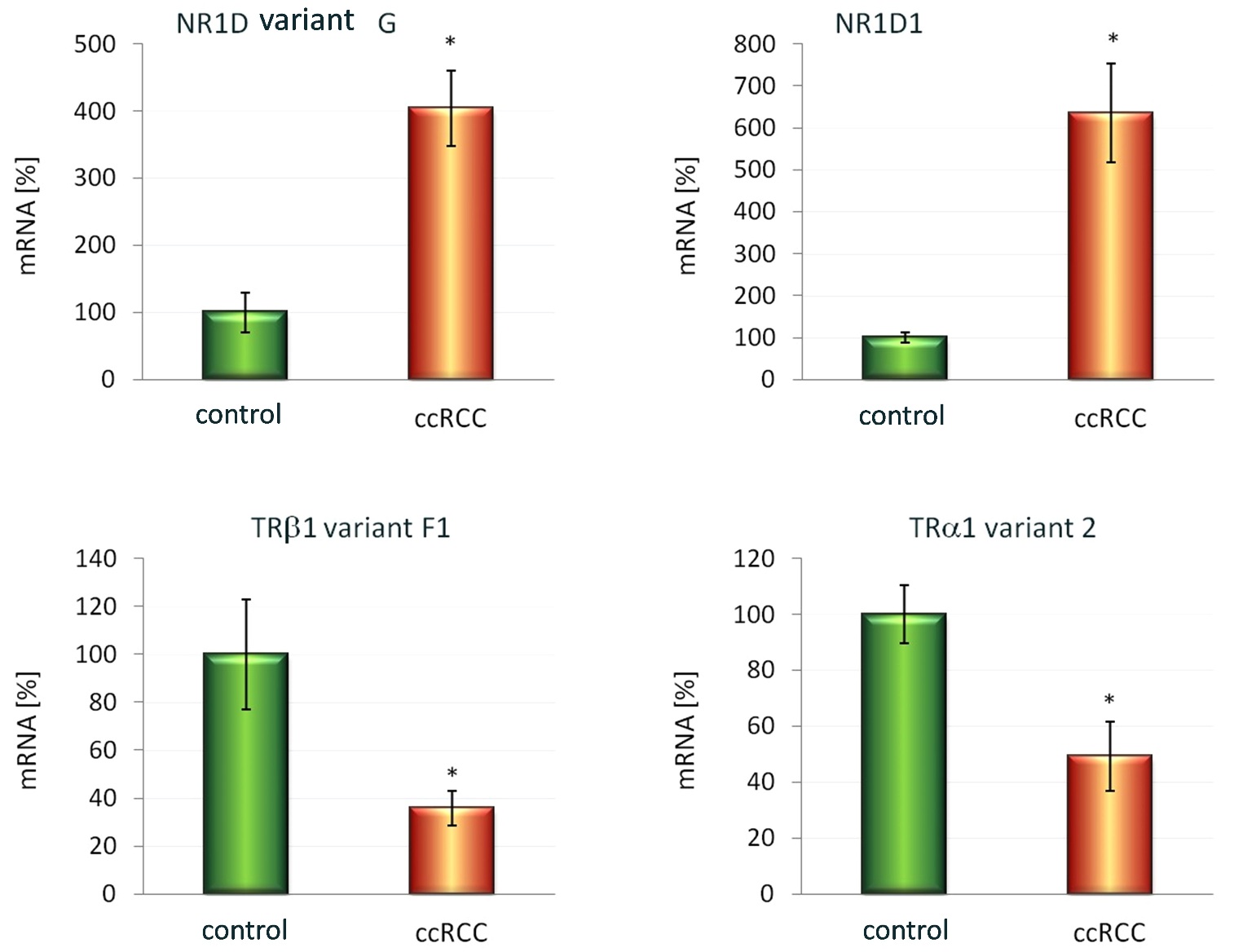
FIGURE 3. Expression of selected mRNA variants encoded by the NR1D1 (B1.) and NR1D2 (A1.) as well as THRB (A2.) and THRA (B2.) genes. The mRNA levels of cis-NATs: Rev-Erbβ variant G (A1) and Rev-Erbα (B1) as well as selected variants of thyroid hormone receptors: TRβ1 variant F1 (B1) and TRα2 (B2) were measured by the use of semi-quantitative Real-Time PCR in clear cell Renal Cell Carcinoma (12 samples assigned as ccRCC) and control samples obtained from the contralateral pole of the same excised kidney with no signs of tumors infiltration (12 samples assigned as Kontrola). These preliminary results revealed in the ccRCCs (when compared to the controls): 4-fold elevation of the Rev-Erbβ variant G (A1. GB.ac.no. CF139138.1); 35% decrease in expression of the TRβ1 variant F1 (A2. GB.ac.no. GQ456950), which 5’ region of pre-mRNA shows partial complementarity with the Rev-Erbβ variant G (antisense transcript); 6-fold increase in mRNA levels of the Rev-Erbα (B1. GB.ac.no. NM_021724.3); and 49% decrease in expression of the TRα2 (B2. GB.ac.no. NM_021724.3), that shows 3’-end complementarity with the Rev-Erbα antisense transcript. Results were normalized to ACTB and HPRT host-gene transcripts. The data from two independent repeats are given as mean ± SEM (n=12 pairs in each repeat). Statistical analysis was performed using the Shapiro-Wilk test followed by the t-student test for two paired groups. *p<0.001 was considered as statistically significant.
Master A, Nauman A.
THRB (Thyroid Hormone Receptor, Beta).
Atlas Genet Cytogenet Oncol Haematol. 2014; 18(6).
This article is available under the following links: XPS (recommended), Article–HTML; Article–PDF; AGCOH no. 06, 2014.
Abstract: Review on THRB, with data on DNA/RNA, on the protein encoded and where the gene is implicated. Identity: HGNC (Hugo): THRB
Alias: C-ERBA-2; C-ERBA-BETA; ERBA2; GRTH; NR1A2; PRTH; THR1; THRB1; THRB2. Organism: Homo sapiens. Location: 3p24.2,chromosome 3, sequence according to the NCBI accession no. NC_000003.11 (24158644..24536772, complement).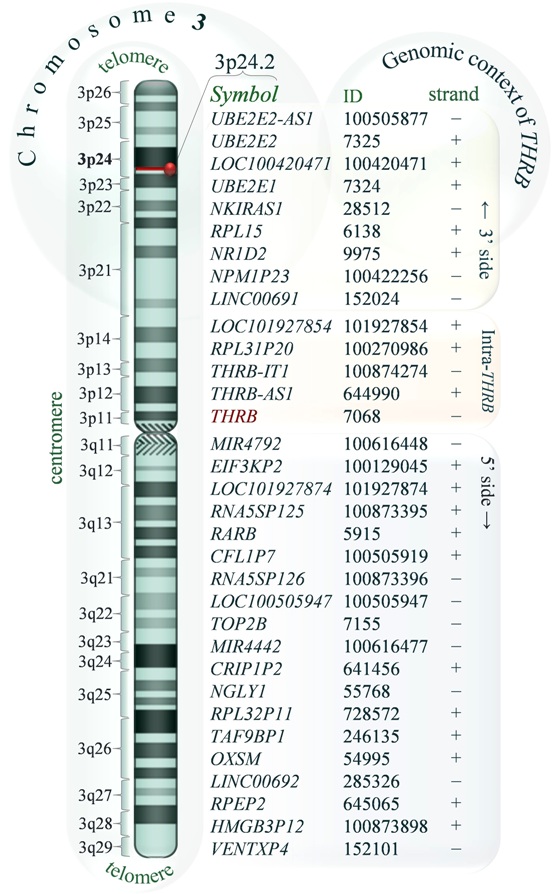 DIAGRAM 1. Schematic representation of human chromosome 3 indicating the position of THRB locus (red bar). The official symbols, NCBI IDs and relative transcriptional orientation of genes in the 3p24.2 locus (+ plus or – minus DNA strand) are shown with respect to the centromere. The genes located at 5’- and 3’-side of the THRB as well as the genes posi-tioned at least in part inside the THRB sequence (in-tra-THRB genes) are highlighted by three coloured rectangles. The diagram was drawn on the basis of the standard ideogram taken from the NCBI Map Viewer and NCBI Human Genome Resources. Local order: According to the NCBI map viewer genes flanking THRB (3p24.2) from telomere to centromere are: UBE2E2-AS1 (UBE2E2-AS1 UBE2E2 antisense RNA 1 (head to head)), UBE2E2 (ubiquitin-protein ligase E2), LOC100420471 (ADP-ribosylation factor-like 4A pseudogene), UBE2E1 (ubiquitin carrier protein E1), NKIRAS1 (NFKB inhibitor interacting Ras-like 1), RPL15 (ribosomal protein L15), NR1D2 (nuclear receptor subfamily 1, group D, member 2), NPM1P23 (LOC100422256, nucleophosmin 1 (nucleolar phosphoprotein B23, numatrin) pseudogene 23), LINC00691 (LOC152024, long intergenic non-protein coding RNA 691), and intra-THRB: LOC101927854 (uncharacterized LOC101927854) sharing some exons with two transcript variants (GeneBank: CB994391.1, AW950510.1) present in ACE View description of NR1D2 gene locus, RPL31P20 (ribosomal protein L31 pseudogene 20), THRB-IT1 (THRB intronic transcript 1), THRB-AS1 (LOC644990, THRB antisense RNA 1), at 5’ side of THRB: MIR4792 microRNA 4792, EIF3KP2 (eukaryotic translation initiation factor 3, subunit K pseudogene 2), LOC101927874 (uncharacterized LOC101927874), RNA5SP125 (RNA, 5S ribosomal pseudogene 125), RARB (retinoic acid receptor, beta), CFL1P7 cofilin 1 (non-muscle) pseudogene 7, RNA5SP126 (RNA, 5S ribosomal pseudogene 126), LOC100505947 (uncharacterized LOC100505947), TOP2B (topoisomerase (DNA) II beta 180kDa), MIR4442 (microRNA 4442), CRIP1P2 (cysteine-rich protein 1 (intestinal) pseudogene 2), NGLY1 (N-glycanase 1), RPL32P11 (ribosomal protein L32 pseudogene 11), TAF9BP1 (TAF9B RNA polymerase II, TATA box binding protein (TBP)-associated factor, 31kDa pseudogene 1), OXSM (3-oxoacyl-ACP synthase, mitochondrial), LINC00692 (long intergenic non-protein coding RNA 692), RPEP2 (ribulose-5-phosphate-3-epimerase pseudogene 2), HMGB3P12 (high mobility group box 3 pseudogene 12), VENTXP4 (VENT homeobox pseudogene 4), see diagram.
DIAGRAM 1. Schematic representation of human chromosome 3 indicating the position of THRB locus (red bar). The official symbols, NCBI IDs and relative transcriptional orientation of genes in the 3p24.2 locus (+ plus or – minus DNA strand) are shown with respect to the centromere. The genes located at 5’- and 3’-side of the THRB as well as the genes posi-tioned at least in part inside the THRB sequence (in-tra-THRB genes) are highlighted by three coloured rectangles. The diagram was drawn on the basis of the standard ideogram taken from the NCBI Map Viewer and NCBI Human Genome Resources. Local order: According to the NCBI map viewer genes flanking THRB (3p24.2) from telomere to centromere are: UBE2E2-AS1 (UBE2E2-AS1 UBE2E2 antisense RNA 1 (head to head)), UBE2E2 (ubiquitin-protein ligase E2), LOC100420471 (ADP-ribosylation factor-like 4A pseudogene), UBE2E1 (ubiquitin carrier protein E1), NKIRAS1 (NFKB inhibitor interacting Ras-like 1), RPL15 (ribosomal protein L15), NR1D2 (nuclear receptor subfamily 1, group D, member 2), NPM1P23 (LOC100422256, nucleophosmin 1 (nucleolar phosphoprotein B23, numatrin) pseudogene 23), LINC00691 (LOC152024, long intergenic non-protein coding RNA 691), and intra-THRB: LOC101927854 (uncharacterized LOC101927854) sharing some exons with two transcript variants (GeneBank: CB994391.1, AW950510.1) present in ACE View description of NR1D2 gene locus, RPL31P20 (ribosomal protein L31 pseudogene 20), THRB-IT1 (THRB intronic transcript 1), THRB-AS1 (LOC644990, THRB antisense RNA 1), at 5’ side of THRB: MIR4792 microRNA 4792, EIF3KP2 (eukaryotic translation initiation factor 3, subunit K pseudogene 2), LOC101927874 (uncharacterized LOC101927874), RNA5SP125 (RNA, 5S ribosomal pseudogene 125), RARB (retinoic acid receptor, beta), CFL1P7 cofilin 1 (non-muscle) pseudogene 7, RNA5SP126 (RNA, 5S ribosomal pseudogene 126), LOC100505947 (uncharacterized LOC100505947), TOP2B (topoisomerase (DNA) II beta 180kDa), MIR4442 (microRNA 4442), CRIP1P2 (cysteine-rich protein 1 (intestinal) pseudogene 2), NGLY1 (N-glycanase 1), RPL32P11 (ribosomal protein L32 pseudogene 11), TAF9BP1 (TAF9B RNA polymerase II, TATA box binding protein (TBP)-associated factor, 31kDa pseudogene 1), OXSM (3-oxoacyl-ACP synthase, mitochondrial), LINC00692 (long intergenic non-protein coding RNA 692), RPEP2 (ribulose-5-phosphate-3-epimerase pseudogene 2), HMGB3P12 (high mobility group box 3 pseudogene 12), VENTXP4 (VENT homeobox pseudogene 4), see diagram.
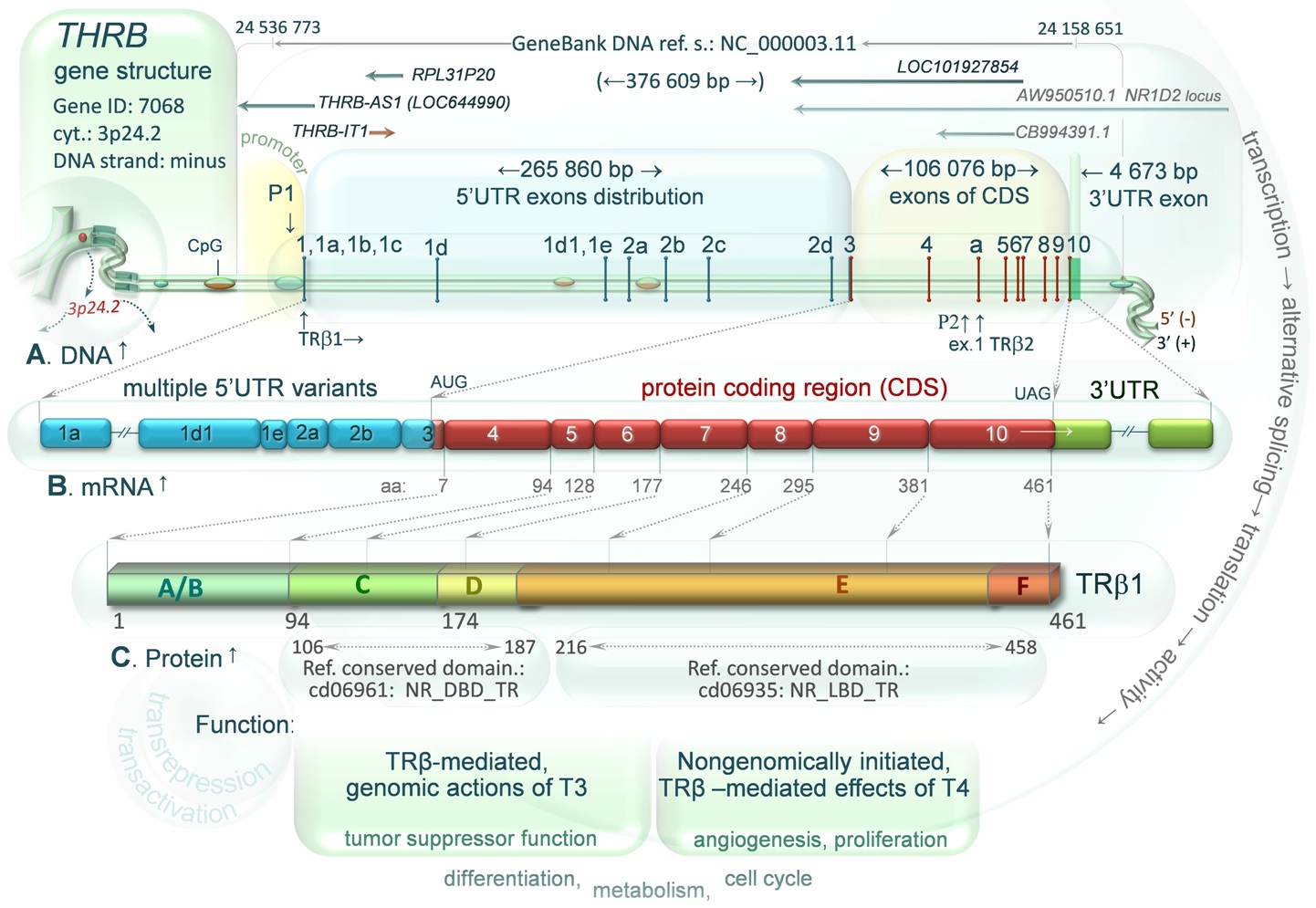
DIAGRAM 2. Human THRB Gene Structure, a transcript and protein. Multiple transcript variants and 3 protein isoforms (TRβ1 TRβ2 and truncated variant TRβ4) are generated by transcription of pre-mRNA using two THRB promoters (P1 and P2 located between exon 4 and 5), alternative splicing of exons marked with numbers 1-10 (horizontal bars) and separated by large introns (shown by distances between exons) as well as protein translation, which is differentially regulated by the 5’UTR variants. A. Genes localized within the THRB sequence are marked with orange or blue arrows. The orange one (THRB-IT1, THRB intronic transcript 1) is transcribed in the same orientation (-) to the THRB, whereas blue arrows indicate the genes in opposite transcriptional orientation (+): THRB-AS1, RPL31P20, LOC101927854, and two transcript variants (GeneBank: CB994391.1, AW950510.1) described in NR1D2 gene locus (see local order). Expression of the genes may result in production of long naturally occurring antisense transcripts (long NATs) that may bind complementary target strands of THRB DNA or newly synthesized pre-mRNA. These sense-antisense pairs may affect the expression of the THRB gene (for more details see diagram 1). Large CpG islands identified as methylated within THRB sequence in human cerebellum, sperm and bone marrow cells are marked with small green ovals on double stranded DNA, whereas the green/orange ovals represent CpG islands identified as methylated in human colon mucosa and colorectal tumor (drawn on the basis of the NCBI Epigenomics database). B. An example of the multiple mRNA variants, wherein blue boxes represent alternatively spliced exons of 5’ untranslated region (5’UTR), red – protein coding sequence, green – TRβ1 3’UTR (exon 10). C. One of two TRβ proteins (here TRβ1) and its functional domains: N-terminal AF1 domain (A/B), DNA binding domain (C), hinge region (D), ligand (T3) binding domain (E) and C-terminal AF2 domain (F), all presented in the context of subsequent amino acids (aa) encoded by the TRβ1 mRNA (CDS). Amino acid ranges of the NCBI reference conserved domains: C and E are indicated by gray arrows. For protein function see the text below.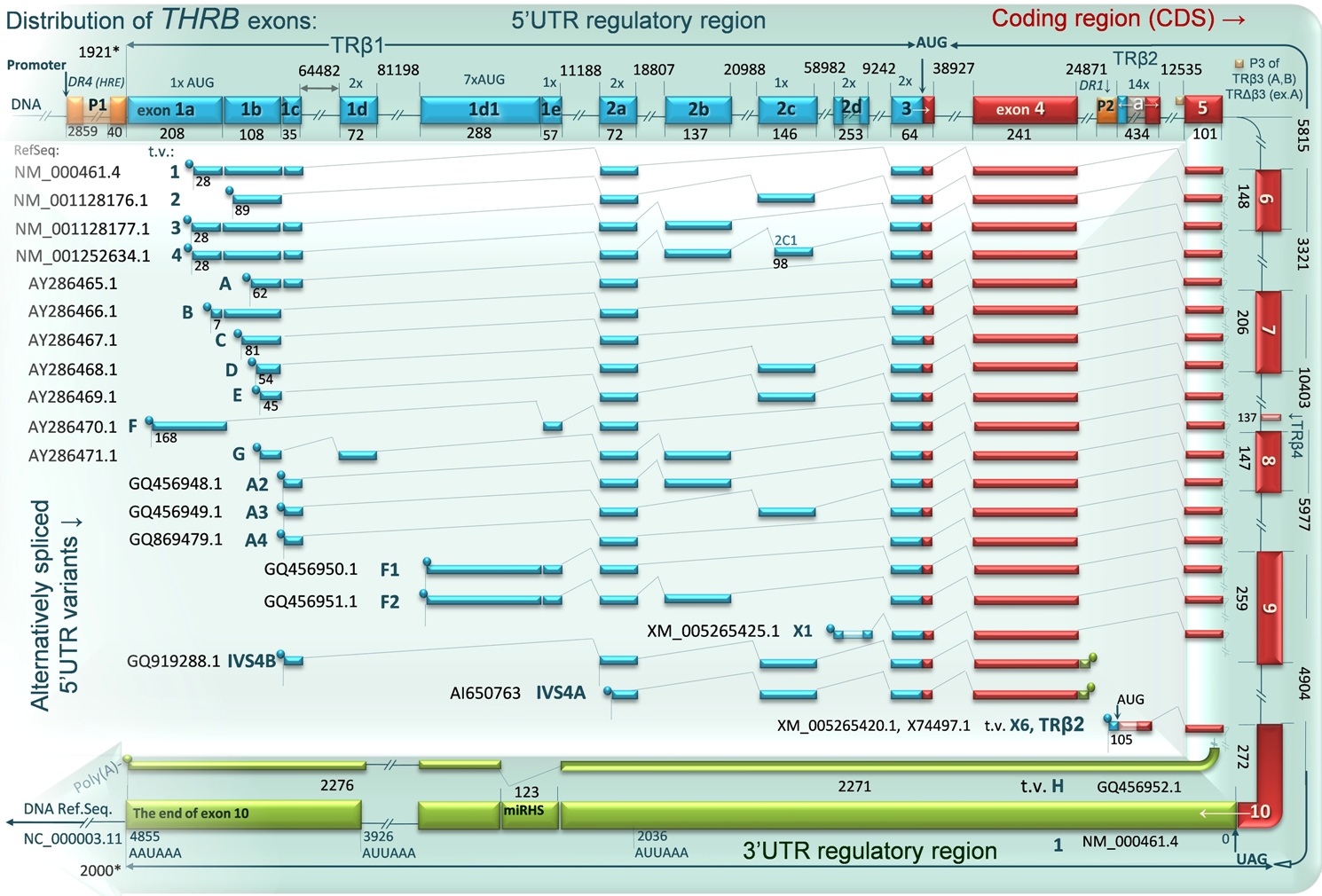
DIAGRAM 3. THRB exons distribution and alternatively spliced 5’UTR variants of TRβ1 TRβ2 and TRβ4 mRNAs. At least 19 mRNA variants of TRβ1 and one of TRβ2 are synthesized using two THRB promoters: P1 and internal promoter P2 located between exon 4 and 5. The human variant TRβ4 is a carboxyl-terminal splicing variant of TRβ1 (using P1 promoter), which contains a stop codon due to the presence of a 137-bp insertion located between exon 7 and 8.The third known, non-functional in humans promoter (P3) is shown as well. This locus is located upstream of the exon 5 but downstream of the P2 promoter and allows for transcription of two additional isoforms: TRβ3 and TRΔβ3, expressed ubiquitously only in rats, however, the regulatory elements present in this region may interfere with transcription of the TRβ2 in humans. The mRNA variants are shown in the context of alternative and constitutive exons and their distribution within the DNA sequence. Blue boxes represent alternatively spliced exons of 5’ untranslated region (5’UTR), red – protein coding sequence, green –3’ untranslated region (3’UTR, the longest 3’ part of exon 10). Most of exons are separated by large introns, which are marked at the beginning and the end by numbers representing their relative position on DNA. The diagram shows a region of 378,609 kb (24133709.. 24516317; Ref.seq. : NC_000003.11). The exact size (nt) of each exon is given below the exon boxes. The 5’UTR region of TRβ1 may include up to 10 alternatively spliced exons and 44 bp of exon 3, whereas 5’UTR of TRβ2 contains only 105 of 434 nucleotides of exon 1 named “a”. The exons 5-10 are mostly constitutive and common with almost all transcripts including TRβ2 mRNA. The most of identified transcript variants (shown at the middle of the diagram) differ only in the 5’ untranslated region, which can influence the protein synthesis. 3’UTR variant H lacks 123 nucleotides (miRHS fragment). This region contains a putative binding site for miRNA-204, located between nucleotides 2313-2319 of TRβ 3’UTR. Variants IVS4A and IVS4B contain alternatively spliced exons of 5’UTR, exon 3, 4 and a fragment of intron with a stop codon located downstream of the exon 4 that may result in translation of truncated protein (28 amino acids) of unknown function. The GeneBank accession numbers for each reference sequence are given next to the adjacent transcript variant.
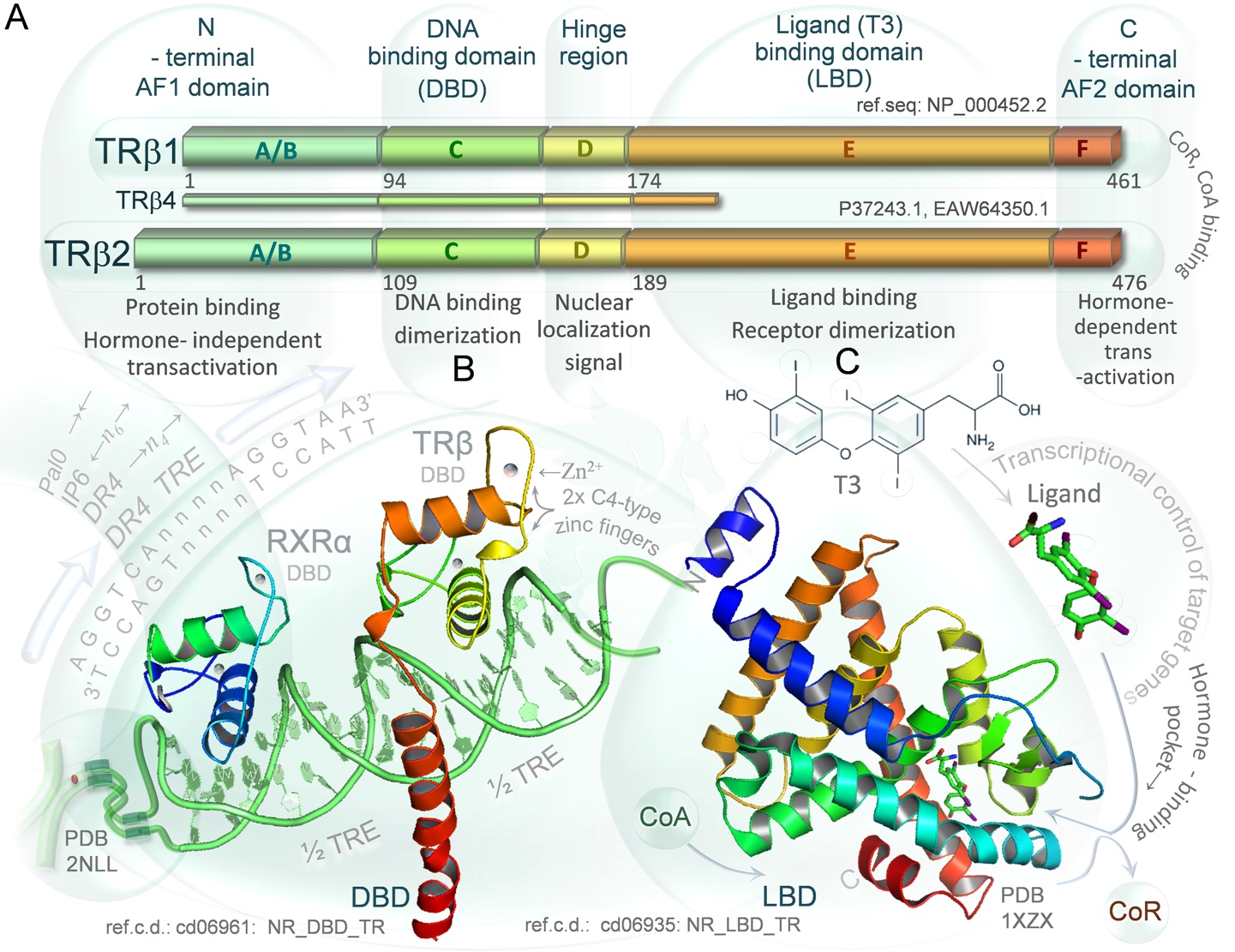
DIAGRAM 4. Structural and functional organization of TRβ proteins. A. Three thyroid hormone receptor beta isoforms: TRβ1 TRβ2 and truncated variant TRβ4 are encoded by the human THRB gene. Functional domains of the transcription factors are divided as follows: N-terminal AF1 domain (A/B) responsible for hormone independent transactivation and regulatory proteins binding; DNA binding domain (C) containing two C4-type zinc fingers; hinge region (D) with nuclear localization signal allowing for nuclear transport (regardless of T3 binding status); ligand (T3) binding domain (E) that allows for dimerization (usually with RXRα receptor) as well; C-terminal AF2 domain responsible for T3-dependent transactivation of target genes (F). The domains E and F bind corepressors or coactivators regulating the activity of the TRβ receptors. The TRβ1 and TRβ2 are functional receptors of T3 differing only in the length of the A/B domain, however their expression is tissue specific. The human variant TRβ4 is a truncated variant of TRβ1, lacks T3 -binding ability and acts as an endogenous dominant-negative isoform B. Crystallographic structure of heterodimer formed by the human thyroid hormone receptor DNA-binding domain and retinoid X receptor DNA-binding domain (rainbow colored) complexed with double strained DNA (green). Zinc atoms of are depicted as blue/pink dots. The structure is presented in the context of the thyroid hormone response elements (TREs) that are specific DNA sequences recognized by the receptors. The TRE-DR4 is formed by consensus core recognition motif (AGGTCA) that is positioned as direct repeats separated by 4 nucleotides. The other TREs including palindrome (TRE-P0) or inverted palindrome (TRE-IP6) are shown as well. C. Crystallographic structure of the ligand binding domain of the human thyroid hormone (T3) receptor beta (rainbow colored, N-terminus in blue, C-terminus in red) complexed with triiodothyronine (T3). Binding of unliganded receptor alone to DNA usually leads to recruitment of corepressors (CoR) and inhibition of target gene basal transcription, whereas binding of T3 in hormone binding pocket is thought to cause conformational changes leading to dissociation of corepressors followed by recruitment of coactivators (CoA) and activation of the transcription. Mutations in C-terminal α-helix domain (red) that can close the hormone binding pocket and the α-helix shown here in green are frequent findings in the thyroid hormone resistance syndromes (RTHs) and cancers. The conserved domains were visualized using PyMOL 1.3 Molecular Graphics System, on the basis of crystallographic structure files (PDB: 2NLL, 1XZX) of The RCSB Protein Data Bank and The NCBI Conserved Domains Database (CDD, ref.c.d.: cd06961 NR_DBD_TR, cd06935: NR_LBD_TR).
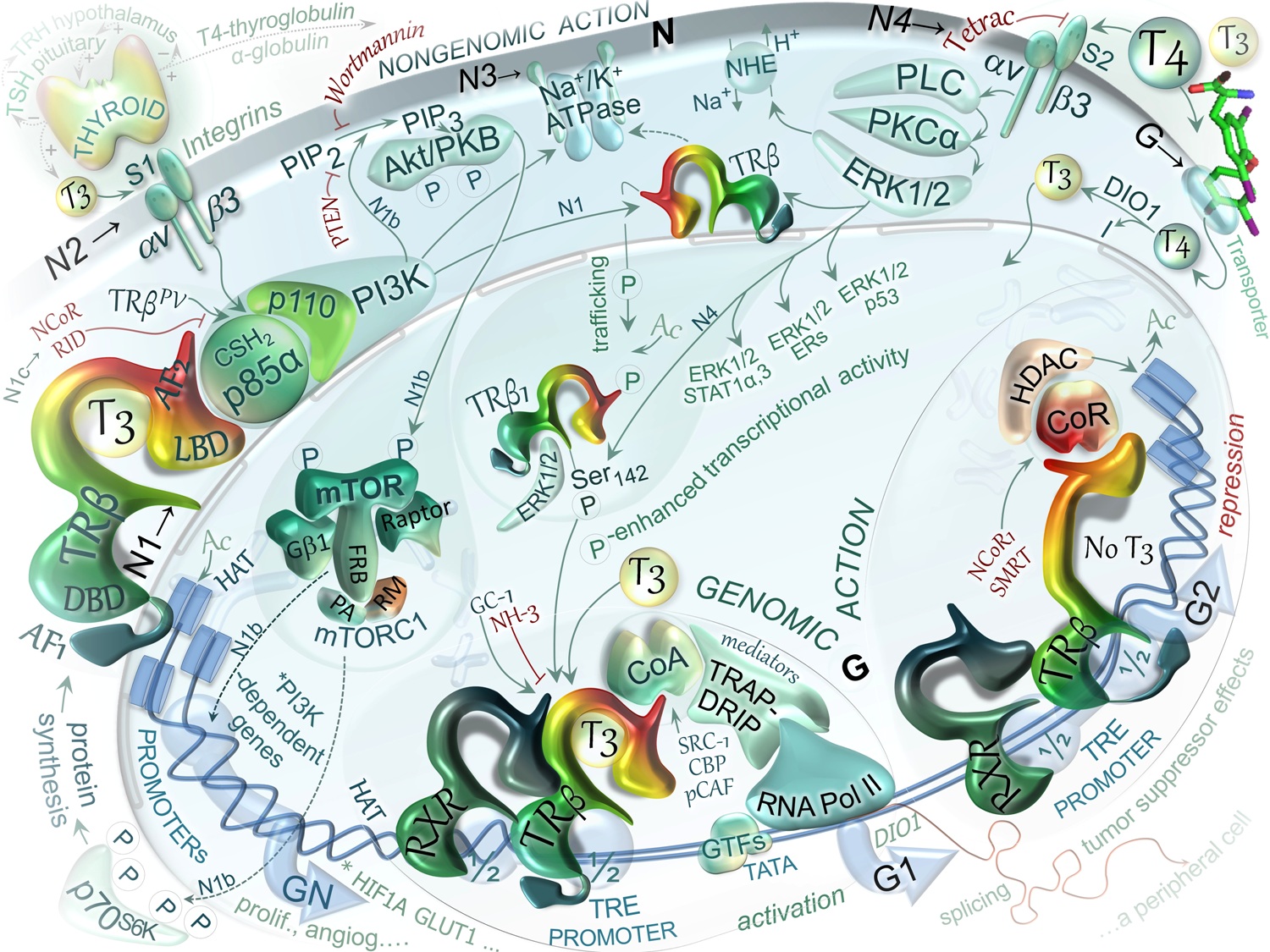
DIAGRAM 5. Schematic representation of selected TRβ-mediated, genomic and non-genomic actions of TH. The thyroid gland, in response to TSH, produces thyroxine (T4) and 3,5,3'-triiodo-L-thyronine (T3), however greater amounts of T4 are produced than T3. The thyroid hormone (TH) in the circulation are bound to protein –transporters that deliver TH to peripheral tissuesues wherein TH triggers TRβ-mediated effects including negative regulation of the hypothalamic-pituitary thyroid (HPT) axis. For genomic actions, T4 needs to be converted to T3 by DIO1 or DIO2 present in peripheral tissues such as liver, brain or kidney. G. Classical model of TH actions in the nucleus. The model is based on the action of triiodothryonine (T3, ligand) on positively regulated genes. The regulation requires: thyroid hormone response elements (TREs) on specific genes, complexes of nuclear TH receptors (TRs) and T3, coactivator (CoA) or corepressor (CoR) nucleoproteins, and histone acetyl transferase (HAT) or deacetylase (HDAC). G1. Ligand-bound state. TRβ can bind to DNA as heterodimer with RXR and regardless of T3 binding status. However, T3 binding results in release of corepressor complex, recruitment of an coactivator complex (e.g. SRC-1, CBP, p300, pCAF, TRAP-DRIP, mediator/integrator components) and HAT. The HAT activity allows for reducing chromatin compaction and permitting general transcriptional factors (GTFs) to interact with DNA and activate transcription of a target gene. G2. Ligand-free state. In absence of ligand, the unliganded TH receptors interact with a copressor complex that may include NCoR, SMRT and histone deacetylase 1 (HDAC1). The recruitment of this complex may result in reduced histone acetylation (shown as Ac), which in turn compacts chromatin structure and represses the gene transcription. The transactivation domain of the T3-free receptor, as a heterodimer with RXR, assumes a conformation that promotes interaction with a group of transcriptional corepressor molecules. N. Nongenomic actions of TH mediated by TRβ1. These actions are fast (within 10-40 min), frequently reported to result in pro-proliferative, pro-angiogenic, anti-apoptotic effects. These actions may be simplified into two main signaling cascades: N4) extracellular-T4/ αvβ3-integrin/ PLC/ PKCα/ ERK1/2/ TRβ1-Ser142 phosphorylation that among others can result in specific gene transactivation or transrepression (N4); N1) cytoplasmic-T3/ TRβ1/ CSH2-p85α-p110(PI3K)/ Akt/ mTOR phosphorylation leading to transcription of PI3K-specific genes such as HIF1A and GLUT1 (SLC2A1) (N1, N1b, N1c). Specific inhibitors of these pathways are shown in red font. N1. Nongenomic effects of T3 may be initiated in cytoplasm by TRβ-dependent activation of PI3K that leads to sequential activation of Akt/PKB/mTOR-p70S6K as well as the other mTOR targets including upregulation PI3K-dependent genes, A fraction of TRβ1 present in the cytosol forms a complex with p85α subunit (regulatory subunit of PI3K) in a ligand independent manner that activate PI3K. The kinase generates phosphatidyl inositol-3,4,5-triphosphate (PIP3) from PtdIns(4,5)P2 (PIP2) activating downstream pathways via Akt/PKB (N1b) or through phosphorylation of the TRβ1 followed by its nuclear import. Wild-type TRβ1 competes with corepressor NCoR or mutant TRβPV for binding to the CSH2 domain of p85α (N1c). This PI3K activity is blocked by specific inhibitors such as Wortmannin or LY294002. N2. Signal transduction via plasma membrane receptor αvβ3 by T3 binding to the extracellular part of the receptor. The binding domain includes a receptor site (S1) exclusively for T3 that activates phosphatidylinositol 3-kinase (PI3K) and leads to shuttling of cytoplasmic TRβ to the nucleus followed by transcription of specific target genes such as HIF1A. N3. Cytosolic TRβ1 and PI3K are involved in T3-stimulated activation of Na+/K+-ATPase and other features of the sodium pump (gene expression, plasma membrane insertion). Besides, TH is known through αvβ3 to modulate the activity of several other ion transport systems including Na+/H+ exchanger NHE (SLC9C1). N4. T4-induced activation of ERK1/2 through plasma membrane receptor αvβ3 (site S2). This action is relevant to: intracellular trafficking of proteins, including TRβ1, serine phosphorylation (P) and acetylation (Ac) of this nuclear receptor, assembly within the nucleus of complexes of coactivators or corepressors and transcription of specific genes, including that for TRβ1. The action includes T4 binding to the extracellular part of the receptor, activation of PLC, PKC, ERK1/2 (MAPK) pathway, phosphorylation on TRβ(Ser142), derepression and enhancement of transcription. Among the consequences of ERK1/2 activation are specific serine phosphorylation of the cytoplasmic/nuclear TRβ1 (Ser142) and estrogene receptor α ERα, phosphorylation of signal transducers and activators of transcription STAT1α, STAT3 as well as p53, wich were found to be co-immunoprecipitated with the activated ERK1/2. Cytoplasmic fraction of TRβ are shuttled to the nucleus, wherein the proteins are transcriptionally active and can modulate the actions of certain cytokines and growth factors including those involved in tumor cell proliferation and on angiogenesis. TETRAC is an analog of T4 that can inhibit this nongenomic action of T4, however, showing thyromimetic properties it can also affect gene expression in the cells, regulating transcription of target genes such as THBS1, CASP2 and CBY1. For details see text.
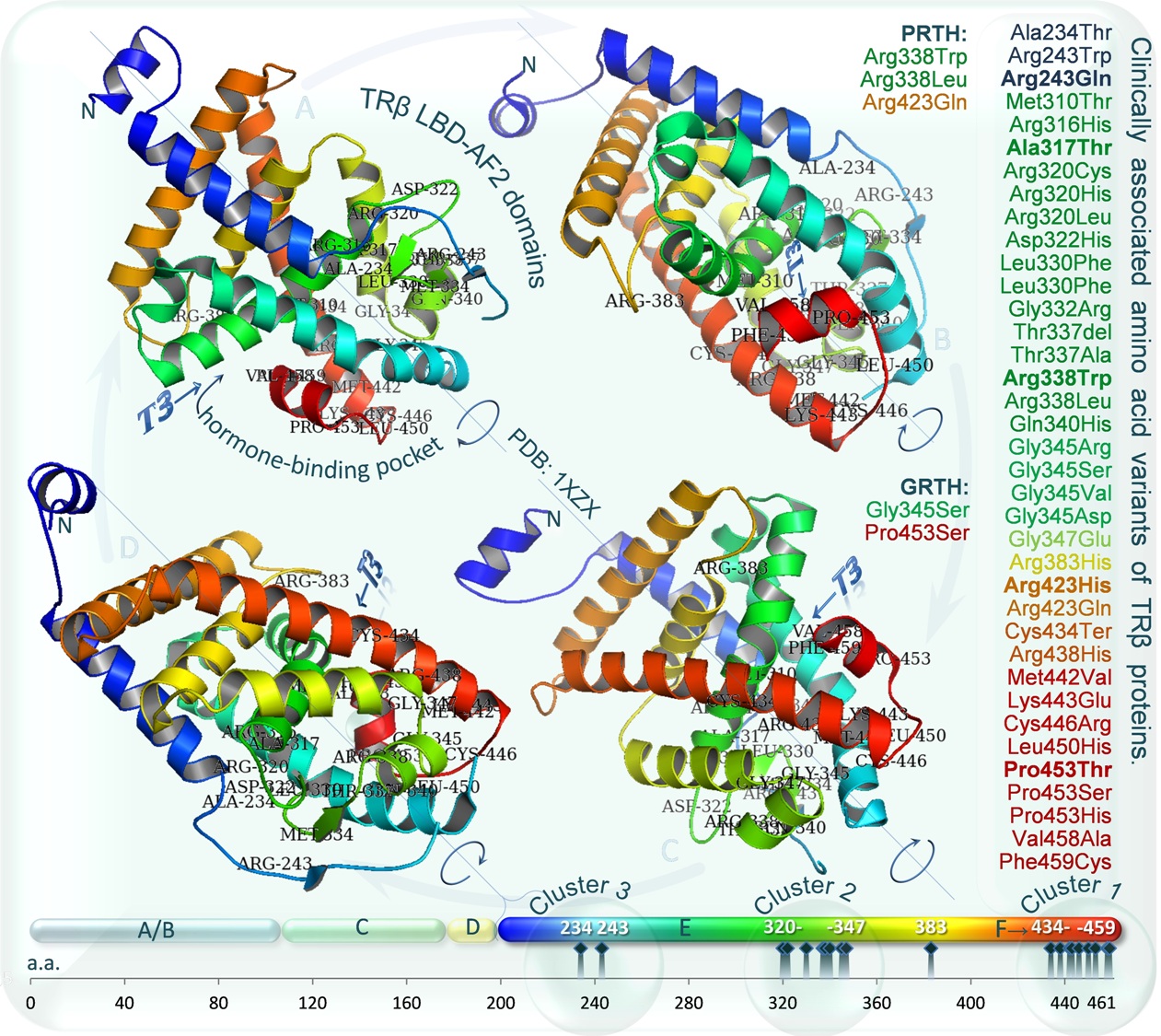
Diagram 6. Clinically asociated amino acid variants of TRβ proteins. Graphic representation of three mutational “hot spots” in the TRβ ligand-binding domain, in which natural mutations have clustered. Crystallographic structure of the TRβ ligand binding domain (LBD, E) complexed with triiodothyronine (T3) and C-terminal domain T3-dependent transactivation (F) are shown on all four sides (A,B,C,D) to visualize listed (on the right) reference variants, which colour corresponds to each α-helix structures of LBD (rainbow colored, N-terminus in blue, C-terminus in red). Mutations in the LBD may be associated with resistance to thyroid hormone (RTH). The most frequent mutations in RTH are bolded. Substitutions associated with pituitary-specific RTH (PRTH, R338L, R338W, R429Q) and generalized RTH (GRTH, P453S, G345S) are given. Mutations of GRTH (P453S, G345S) impair both TRβ2 and TRβ1 function proportionally, whereas variants of PRTH disproportionately disrupt the function of TRβ2 (Wan et al., 2005). An increased inability of the mutants to properly release the nuclear corepressors is postulated to inhibit the T3-mediated transactivation or transrepression of target genes. The TRβ mutants function in a dominant-negative fashion to interfere with the transcription activity of other wild-type thyroid hormone receptors (TRα) leading to resistance in peripheral tissues and dysregulation of the hypothalamic-pituitary thyroid axis (Dumitrescu and Refetoff, 2013). The conserved T3 binding domain was visualized using PyMOL 1.3 Molecular Graphics System, on the basis of crystallographic structure file (PDB: 1XZX) of the RCSB Protein Data Bank and The NCBI Conserved Domains Database (CDD, ref.c.d.: cd06961, NR_LBD_TR). For a more extensive listing of mutations, see recerences.
.jpg)


